Water evaporates when heated. This is basic physics that anyone who owns a stove or clothes dryer can attest to.
As water temperature increases, the energy of water molecules increases. This extra force is enough to stir up molecules, breaking the bonds between them, causing some to drift into the atmosphere as vapor. Of course, there’s more to it, but that’s essentially how the kettle boils.
But according to a recent study, that may not be the only way to evaporate water. A team of MIT researchers has found evidence that light itself (without heat) may also cause water to evaporate. If accurate, this strange discovery could represent a new natural phenomenon.
A new look at evaporation
While exploring solar evaporation, MIT postdoc Yaodong Tu and mechanical engineering professor Gang Chen noticed some unexpected results in their experimental studies. Some hydrogel experiments produced water evaporation that exceeded the thermal limit (the expected amount of evaporation given the amount of heat introduced). In some cases, evaporation has even doubled.
After looking more closely at the data, Tu, Chen and their team suspected that light itself might be causing the difference.
Now, light does help water evaporate. For example, the reason dew disappears in the morning is because the sun heats it throughout the day. However, the idea that the wavelength of light itself might break the bonds of water molecules is new.
To test their hypothesis, the researchers exposed water-soaked hydrogels to different colors of light. These hydrogels were placed on a scale so that the amount of water lost could be measured directly. They also use light shades to reflect heat and monitor temperatures closely.
They found that light indeed produced evaporation beyond the thermal limit. The effect also varies by wavelength – green light produces the most evaporation.
The researchers believe that since the wavelength difference is not related to heat, this supports the hypothesis that light is the cause. They also double-checked their results by repeating the experiment without light. Instead, they heated the hydrogel to the same temperature as in the light experiment. However, under these conditions, the amount of water evaporated is much less.
“We tested it under a solar simulator and it worked,” Chen said. “So, we believe [the other experiments] Now. “
Researchers publish their findings in peer review Proceedings of the National Academy of Sciences.
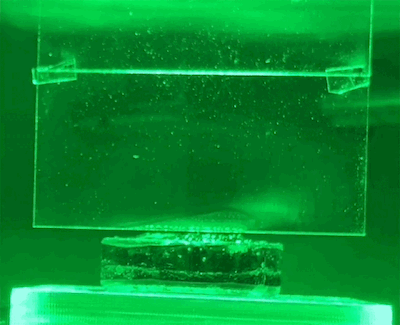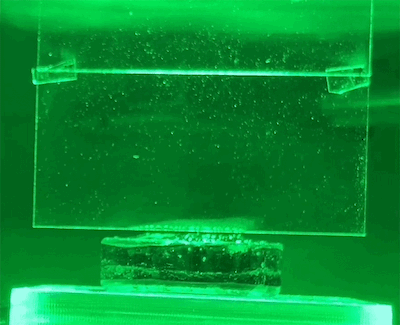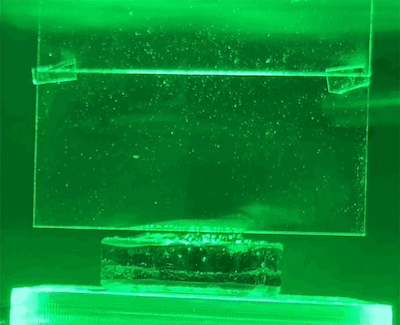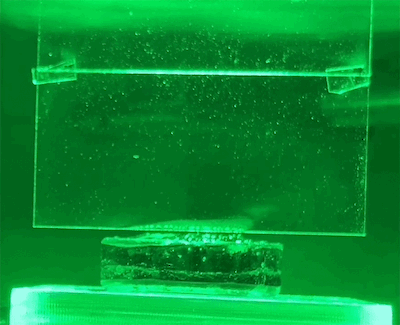
New physics?
While light appears to cause increased evaporation, researchers don’t yet know why. They believe that light particles (photons) may loosen clusters of molecules on the surface of the water.
“It’s very convincing that in this particular experimental setup, you can see clumps of molecules falling off and then those clumps evaporating,” said Janet Elliott, a thermodynamicist at the University of Alberta. , she was not involved in this study. science news.
This is because during thermally driven evaporation, molecules escape one by one. This distinction is another reason why MIT researchers believe they may have discovered a unique phenomenon they call the “photomolecular effect.”
That said, Elliott noted that there are still many questions that must be answered before determining whether the proposed effects are real. For example, researchers don’t know how photons break water’s bonds. They also don’t know why green light works better, or whether the effect occurs in nature—for example, on the surfaces of lakes and clouds—or if it’s unique to the soaked hydrogel used by the MIT researchers some.
Full speed ahead
The team is currently working to answer these questions and, in coordination with other researchers, hopes to replicate the findings and build a more robust theoretical framework. If further experiments show that the hypothesis is correct, the next step will be to determine where in nature it may occur and the extent to which it affects natural processes.
“This photomolecular evaporation process may occur widely in nature. It may have a significant impact on the Earth’s water cycle [and] climate change,” the researchers wrote.
They are also investigating potential applications for this effect. They received funding to study how it changes climate models and how solar-powered desalination systems can be improved. Chen calculated that if their discovery proves feasible, the efficiency of the desalination process could increase by three to four times.
“This could really lead to cheap desalination,” he said.
#Light #water #evaporate #heat
Image Source : www.freethink.com
