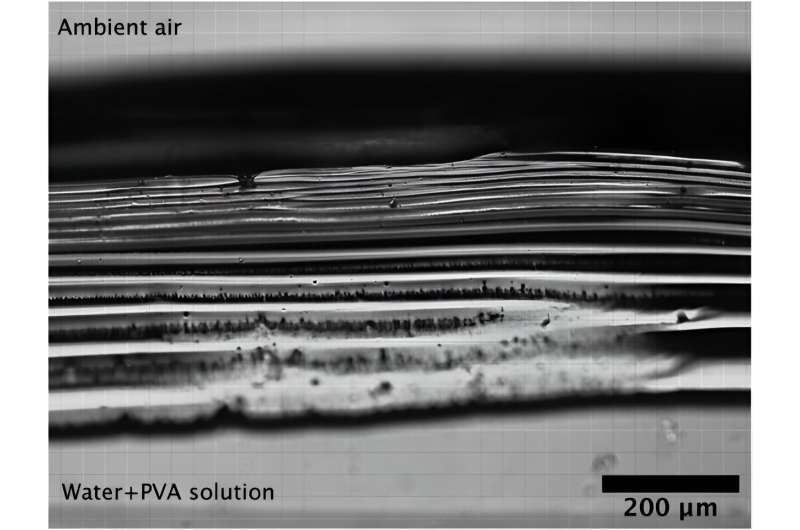
Brightfield image of the polarizing layer at the terminal water-air interface (t103minutes) typical experiment (RH=50%). Credit: Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.248102
A team of physicists from the University of Edinburgh, working with infection and immunity experts at the university’s Roslin Institute, have experimentally tested a theory that explains why paint dries at the same rate regardless of humidity levels.The study was published in Physical Review Letters.
Common sense dictates that paint on an outdoor fence should dry faster on dry days than on humid days because evaporation occurs faster when the air around the source of the liquid dries. But anecdotal evidence suggests that’s not the case with paint and other liquids. Six years ago, chemist Jean-Baptiste Salmon and colleagues proposed a theory to explain why this happens. They believe this is because macromolecules in the liquid are pulled to the surface during the evaporation process, forming a “polarized layer” that inhibits evaporation and thus inhibits drying. In this new effort, the team conducted an experiment to test this theory.
The researchers drilled five holes into a squat, round cylinder and inserted glass capillaries in a horizontal position, then sealed each hole in place. They then added a certain amount of polyvinyl alcohol to the graduated cylinder and placed it on a scale. They poured a thin layer of oil on top of the liquid to prevent evaporation from the surface.
The final step is to place a humidity control airflow box on top of the cylinder to control humidity levels. The team then conducted multiple 17-hour experiments to determine the tube’s evaporation rate (using a scale to measure the amount of liquid evaporated) at different humidity levels, ranging from 25% to 90%.
The researchers found that, as expected, the evaporation rate remained constant for about three hours. But then, as Salmon surmised, the ratio plummeted regardless of humidity levels. During the first three hours, the evaporation rate does not decrease as humidity increases. However, this theory only seems to hold true at humidity levels up to 80% and the rate above that level, where evaporation does slow down, which the team believes may be due to some other force.
The researchers say their work may have medical applications, as recent work has shown that respiratory droplets tend to form on the skin similar to those seen in the experimental setup.
More information:
Max Huisman et al., Evaporation of concentrated polymer solutions is insensitive to relative humidity, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.248102
2023 Science X Network
citation: Why Paint Doesn’t Dry Slower in Humid Environments (2023, December 20), Retrieved December 21, 2023, from https://phys.org/news/2023-12-dry-slower- humid-environment.html
This document is protected by copyright. No part may be reproduced without written permission except in the interests of fair dealing for private study or research purposes. Content is for reference only.
#paint #doesnt #dry #slower #humid #environments
Image Source : phys.org