For centuries, goldsmiths have searched for ways to flatten gold into finer shapes. Methods based on modern chemistry ended up creating a gold material composed of a single layer of atoms that is almost impossible to get thinner.
Following naming conventions in materials science, the researchers named the new two-dimensional material “gold,” which has some interesting properties not found in three-dimensional forms of gold.
“If you make a material very thin, extraordinary phenomena like graphene can happen,” explains Shun Kashiwaya, a materials scientist at Limping University in Sweden.
“The same thing happens with gold. As you know, gold is usually a metal, but if it’s a single atomic layer thick, gold can become a semiconductor.”
Since gold tends to clump together, it is difficult to induce into a two-dimensional structure. Previous attempts either resulted in flakes a few atoms thick or a single layer sandwiched between or on top of another material that could not be separated.
Kashiwaya and his colleagues didn’t set out to make gold, but stumbled upon the first step in their process.
“We had completely different applications in mind when we created the basic materials,” says Lars Hultman, a materials physicist at Linkping University.
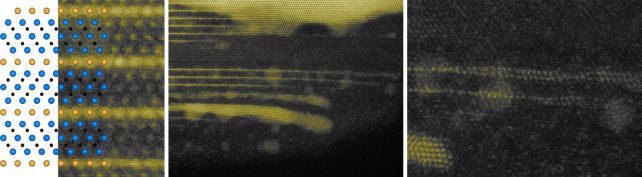
“We started with a conductive ceramic called titanium silicon carbide, in which the silicon is in a thin layer. The idea then was to coat the material with gold to create contacts. But when we exposed the component to high temperatures, the silicon layer was The replacement is made of gold within the base material.
So far, so good. But as with other attempts to create single layers of gold, progress has stalled at this critical step. For several years, the intercalated titanium-gold carbide the team created had been unable to extract an ultra-thin layer of gold from between the titanium and carbon layers.
That’s where a technique based on an etching solution called Murakami’s reagent comes in.
Murakami’s reagent is a mixture of chemicals used in metalworking that is used to etch away carbon and stainless steel, creating the pattern seen on some Japanese knives.
They tried different concentrations of the mixture and different times of the etching process to attack the surrounding titanium and carbon. gold. The longer they sit, the better, but that’s not all the recipe requires.
The etching action of Murakami’s reagent produces a by-product called potassium ferrocyanide. If exposed to light, the compound releases cyanide, which dissolves the gold, so the etching process must be performed entirely in the dark.
Finally, thin gold flakes have a tendency to curl and clump, a problem solved by adding a surfactant that prevents the gold layer from folding and sticking to itself, thus maintaining the integrity of the single layer. Further analysis showed that these tedious steps ultimately succeeded in forming a stable gold color, as predicted by theoretical simulations.
In general, gold is an excellent conductor of electricity. When an element takes the form of a two-dimensional sheet, the atoms have two free bonds, turning it into a semiconductor with conductive properties between a conductor and an insulator. These are useful because their conductivity can be adjusted.
Gold already has properties that make it highly prized for chemical applications. Giving it semiconductor properties also opens up a whole new range of ways for us to use it, including water purification, communications and chemical production.
The team’s research results were published in Natural synthesis.
#Strange #form #gold #exists #flakes #atom #thick
Image Source : www.sciencealert.com